Peptoids
Page Content being updated Jan 2026
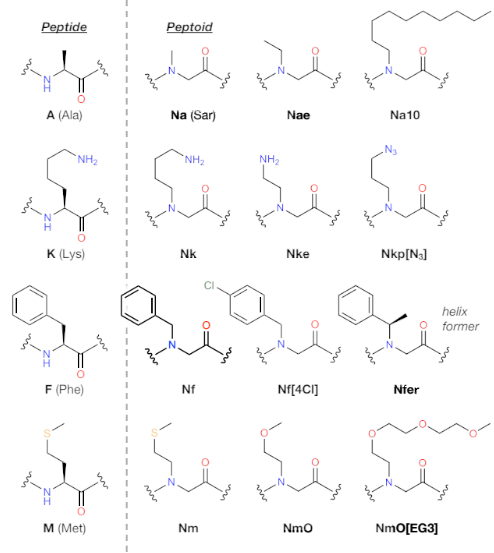
Figure 1. Comparison of peptoid and peptide chemical structures.
Poly(N-substituted glycine) “peptoids”, a class of highly customizable peptidomimetic macromolecules, could potentially enable significant advances. The biorecognition abilities of peptoids have been demonstrated through two decades of combinatorial sequence discovery and secondary structure design. Many bioactive sequences have been discovered by screening peptoid libraries.
In contrast, my research takes advantage of the synthetic accessibility and sequence programmability of peptoids for biointerfaces, self-assembly, and materials and nanostructure engineering. Attention is focused not only on incorporating chemical functionality, but also on exploiting the mainchain flexibility of the peptoid backbone, and the potential of organizing molecular functionality on the nanoscale.
Peptoid Nomenclature
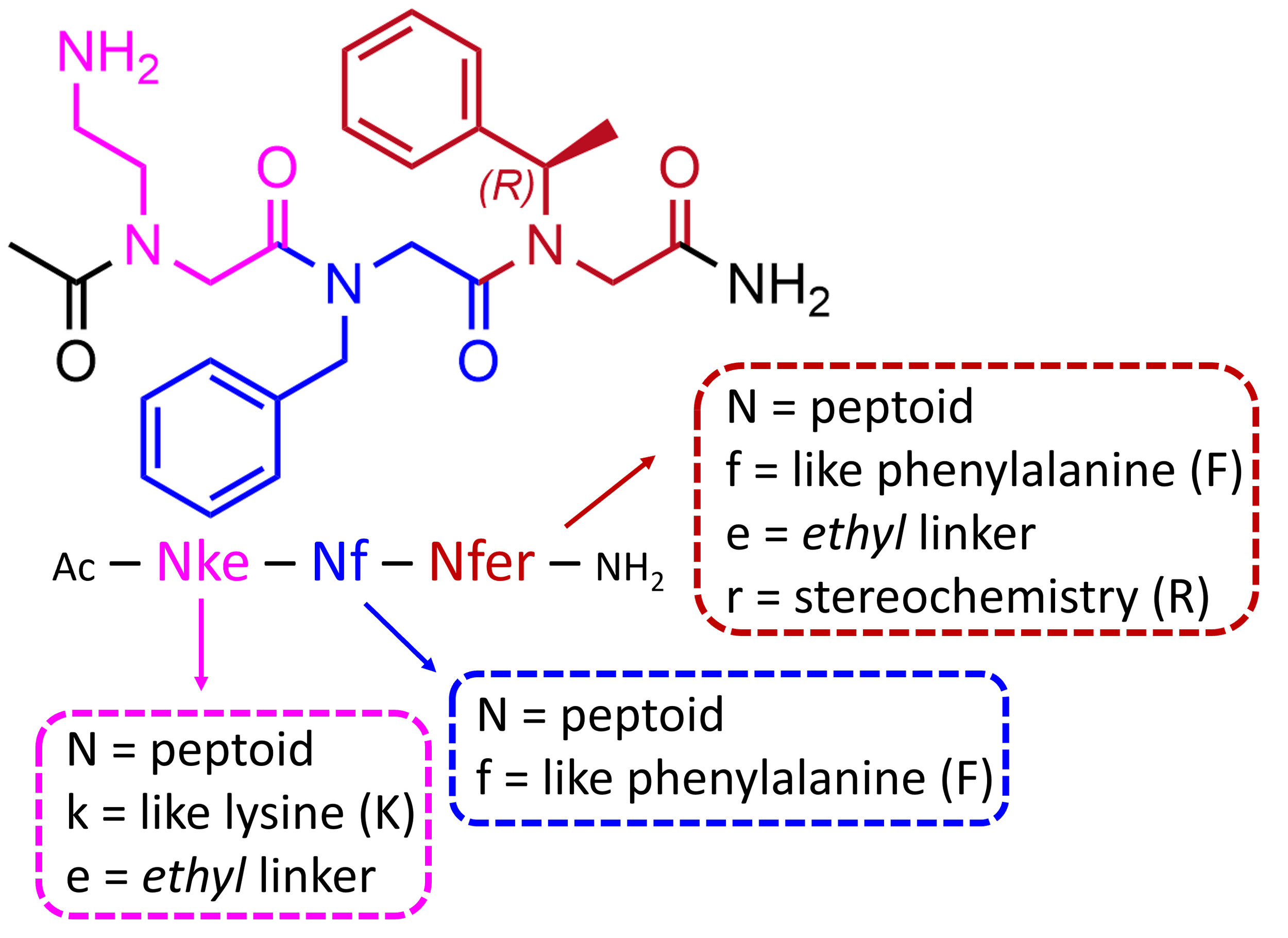
Peptoid residues are generally named with a short form starting with “N,” emphasizing the N-substitution, followed by letters indicating the sidechain chemistry. For example, the analog of the amino acid lysine has appeared as “Nab” or “Nlys,” emphasizing either its aminobutyl chemical structure or its correspondence to lysine indicated by its three-letter code (Figure 2). There is, however, no agreed nomenclature, especially for diverse “nonnatural” sidechains. Therefore, we have recently proposed a naming convention appealing to both the broad knowledge of natural amino acids and a flexible encoding of peptoid sidechain chemistry, as illustrated in Figure 2. The convention of starting with “N” is preserved. This is then followed by characters specifying the sidechain chemistry, with the first position indicating the single-letter code of the most closely related amino acid in small letters. So Nlys is simply Nk. The second position encodes the most characteristic modification, often a different sidechain length change or an atom substitution, e.g. changing Nk to an ethyl connection to the sidechain functional group gives Nke, and substituting the sulfur atom in the methionine analog Nm with oxygen gives NmO. Additional characters then specify further chemical details. The proposed naming scheme is published online for crowd-sourcing suggestions for improvements and standardization. Not yet included in this scheme are classes of sidechains with no analogy to natural amino acids, for which additional characters may be used in the first position after "N". In any case, the aim is to aid researchers in identifying the chemical characteristics of peptoid residues regardless of familiarity with the literature. Peptoids described in this chapter will appear with this naming scheme.
Figures 2.1(left), and 2.2(above)
Direct analogs of peptides and peptoids differ in sidechain position, chirality, and backbone hydrogen presentation. The examples shown illustrate the diversity of sidechain modifications possible and how simple peptoid residues can be systematically named using the single letter code of natural amino acids as a stem in the nomenclature
“30 Years of Peptoids Research”
Introductory seminar given by inventor of modern peptoids Ron Zuckermann (Lawrence Berkeley National Laboratory, USA).
Recorded at the inaugural symposium of the International Online Peptoid Seminar series, October 27, 2020 (Organizers: Aaron Lau (University of Strathclyde), Caroline Proulx (NC State), Jiwon Seo (Gwangju Institute of Science and Technology), Sophie Faure (Chemistry Institute of Clermont-Ferrand), Steven Cobb (Durham University)